In the vast landscape of scientific understanding, few concepts are as foundational as the pH scale. This logarithmic measurement serves as a cornerstone in chemistry, allowing us to gauge the acidic or alkaline nature of substances. One substance that often takes center stage in discussions about what is the ph of distilled water. In this comprehensive exploration, we’ll delve into the nature of the pH scale, uncover the secrets of distilled water’s neutrality, and understand why it matters.
The pH Scale: A Snapshot of Acidity and Alkalinity
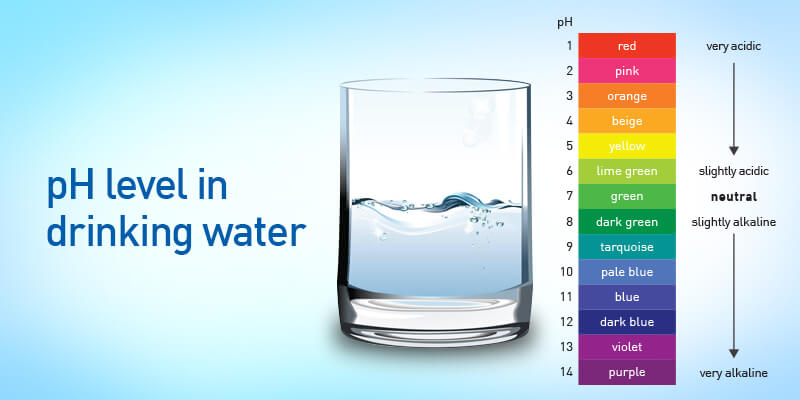
Understanding the pH scale is akin to holding a key to the intricate world of chemistry. Ranging from 0 to 14, this logarithmic scale quantifies the concentration of hydrogen ions in a solution. A pH value of 7 is deemed neutral, indicating an equal balance between acidity and alkalinity. Values below 7 signify increasing acidity, while those above 7 indicate heightened alkalinity.
Unpacking pH Levels
- pH 0-6: Acidity Dominates Substances with pH values below 7 are considered acidic. The lower the pH, the higher the concentration of hydrogen ions, giving rise to the distinct sour taste commonly associated with acidic substances.
- pH 7: Neutrality Prevails A pH of 7 signifies a neutral substance, possessing an equal number of hydrogen ions and hydroxide ions. Distilled water’s pH level lies right here, making it a central point of discussion.
- pH 8-14: Alkalinity Takes the Lead As the pH value surpasses 7, the solution becomes increasingly alkaline. The higher concentration of hydroxide ions imparts a bitter taste to alkaline substances.
Distilled Water: The Neutral Marvel
At the heart of this discourse is distilled water, an enigma in the world of pH. Distilled water, by its very nature, should be neutral, right? Indeed, it is. The process of distillation involves heating water to its boiling point, causing it to evaporate and then condense back into liquid form. This leaves behind impurities, minerals, and ions, resulting in an almost pristine water sample.
The Role of Impurities
Water, as it exists in nature, rarely reaches a true neutral pH. This is often due to the presence of dissolved gases, such as carbon dioxide, which can slightly tip the pH towards the acidic side. However, during distillation, these impurities are left behind, allowing distilled water to come tantalizingly close to that coveted pH of 7.
Distilled Water and pH 7
Distilled water’s neutrality on the pH scale is a testament to its purity. The absence of significant concentrations of hydrogen ions or hydroxide ions means that distilled water remains steadfastly neutral, ready to interact with various substances without altering their pH levels.
The Significance of Distilled Water’s Neutrality
In both scientific and practical contexts, distilled water’s neutrality plays a pivotal role:
Laboratory Applications
In laboratories, precision is paramount. Researchers and scientists rely on distilled water to maintain the integrity of their experiments. Its neutral pH ensures that it won’t interfere with the reactions they are studying, providing a blank canvas for accurate results.
Medical and Pharmaceutical Uses
The neutrality of distilled water extends to medical and pharmaceutical fields. From diluting medications to preparing sterile solutions, its neutral nature guarantees that it won’t introduce variables that could impact patient outcomes.
Industrial Processes
Industries also benefit from the neutral properties of distilled water. Whether it’s for cooling systems, manufacturing processes, or cleaning operations, the absence of extreme acidity or alkalinity helps prevent corrosion and other undesirable effects.
Conclusion
As we navigate the complex landscape of pH, distilled water shines as a beacon of neutrality. Its role as a blank canvas for scientific experiments, a cornerstone in medical applications, and a reliable resource in industries cannot be overstated. From the subtle intricacies of the pH scale to the remarkable purity achieved through distillation, the neutral nature of distilled water underscores its significance in various aspects of our lives. Next time you encounter this unassuming liquid, remember that within its neutral pH lies a world of possibilities.
